Abstract
Background: A key component of maintenance chemotherapy for pediatric ALL is weekly oral methotrexate (MTX), typically given at 20 mg/m2/dose. Another component is steroid/vincristine pulses although there is uncertainty regarding their need or their optimal frequency. AALL0932 was designed to optimize maintenance therapy by asking two questions: (1) the potential superiority of a starting weekly oral MTX dose of 40 mg/m²/dose as compared to the standard 20 mg/m2/dose; and (2) whether giving vincristine/dexamethasone (VCR/DEX) pulses every 12 weeks was non-inferior to every 4 week pulses. Both oral MTX arms used identical dose modifications to adjust dose based on blood counts.
Methods: AR patients had SR B-ALL without CNS3 or testicular leukemia, unfavorable genetic characteristics or Down syndrome, AND had either favorable genetics (presence of trisomies of chromosomes 4 &10 or ETV6/RUNX1 fusion) with Day 8 peripheral blood (PB) minimal residual disease (MRD) ≥ 0.01% or CNS2 status, Day 29 bone marrow (BM) MRD less than 0.01%, or if neutral cytogenetics, had Day 8 PB MRD less than 1% and Day 29 BM MRD less than 0.01%. AR patients were randomized to 1 of 4 Maintenance regimens using a 2 x 2 factorial design: (A): VCR/DEX pulses every 4 weeks and oral MTX 20 mg/m²/week (standard arm); (B): VCR/DEX pulses every 4 weeks and oral MTX 40 mg/m²/week; (C): VCR/DEX pulses every 12 weeks and oral MTX 20 mg/m²/week; and (D): VCR/DEX pulses every 12 weeks and oral MTX 40 mg/m²/week. Between 2010 and 2015, 2366 AR ALL patients were randomized at the start of maintenance to MTX 20 mg/m²/week [Arms A and C (n=1186)] or 40 mg/m²/week [Arms B & D (n=1180)] starting dose.
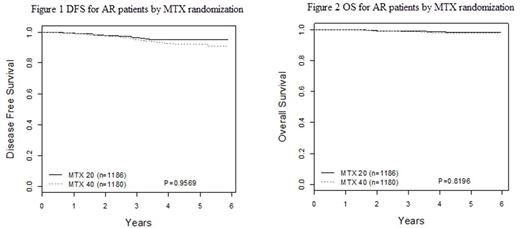
Results: Interim monitoring of COG AALL0932 concluded that a futility boundary had been crossed and that the study would not be able to demonstrate that oral MTX 40 mg/m²/week is superior to 20 mg/m²/week. The 5-year disease-free survival (DFS) estimates for AR patients who received oral MTX 20 mg/m²/week vs. 40 mg/m²/week are 95 ± 2.4% vs 92.3 ± 2.9%, respectively (p=0.95). Corresponding 5-year overall survival (OS) rates are 98.2±1.5% vs. 97.5±1.7%, respectively (p=0.82). Results from the VCR/DEX pulse randomization remain blinded.
Conclusion: Patients with average risk ALL, which accounts for a third of all children with ALL, have outstanding outcomes with 5-year DFS/OS rates of 95% and 98% with standard oral MTX dosing during maintenance therapy. Escalation of the MTX starting dose does not improve outcome.
Zweidler-McKay: ImmunoGen: Employment. Borowitz: Beckman Coulter: Honoraria; HTG Molecular: Honoraria; Becton-Dickinson Biosciences: Research Funding. Hunger: Novartis: Consultancy; Amgen: Consultancy, Equity Ownership; Erytech Pharmaceuticals: Consultancy; Jazz Pharmaceuticals: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal